1000 Μg Ml To Ppm
thedopedimension
Sep 21, 2025 · 6 min read
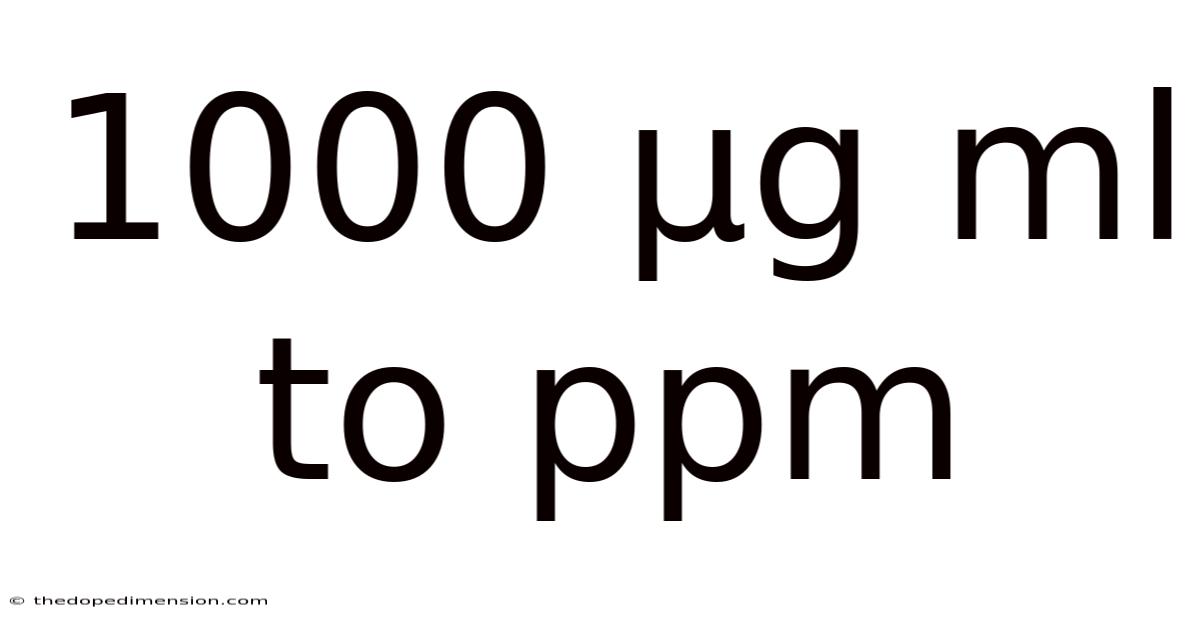
Table of Contents
Decoding the Conversion: 1000 µg/ml to ppm
Understanding unit conversions is crucial in many scientific and engineering fields. This article delves into the conversion of 1000 µg/ml (micrograms per milliliter) to parts per million (ppm), a frequently encountered task in analytical chemistry, environmental science, and toxicology. We'll break down the process step-by-step, providing a clear and comprehensive explanation suitable for both beginners and those needing a refresher. This will include the underlying scientific principles, practical applications, and frequently asked questions to solidify your understanding.
Understanding the Units
Before diving into the conversion, let's define the units involved:
-
µg (microgram): A unit of mass equal to one millionth of a gram (1 µg = 10⁻⁶ g). It's a very small unit, often used for measuring trace amounts of substances.
-
ml (milliliter): A unit of volume equal to one thousandth of a liter (1 ml = 10⁻³ L). It's commonly used to express the volume of liquids.
-
µg/ml (micrograms per milliliter): This expresses the concentration of a substance in a solution. It indicates the number of micrograms of the substance present in one milliliter of the solution.
-
ppm (parts per million): This is a dimensionless unit expressing the concentration of a solute in a solution or a mixture. It represents the number of mass units of solute per one million mass units of solution. While often used for mass ratios, it can also apply to volume ratios in specific contexts, depending on whether the solution is considered incompressible (aqueous solutions, for example)
The Conversion Process: 1000 µg/ml to ppm
The conversion of 1000 µg/ml to ppm hinges on the understanding that 1 ppm is equivalent to 1 mg/L (milligram per liter). This equivalence arises because:
- 1 mg = 1000 µg
- 1 L = 1000 ml
Therefore, we can create a conversion factor:
1 ppm = 1 mg/L = (1000 µg) / (1000 ml) = 1 µg/ml
This equivalence holds true for dilute aqueous solutions where the density is approximately 1 g/ml. For solutions with significantly different densities, a density correction may be necessary, as we will see later.
Step-by-step conversion of 1000 µg/ml to ppm:
-
Start with the given concentration: 1000 µg/ml
-
Use the conversion factor: Since 1 µg/ml = 1 ppm (for aqueous solutions with density ≈ 1 g/ml), we can directly substitute:
1000 µg/ml = 1000 ppm
Therefore, 1000 µg/ml is equivalent to 1000 ppm.
Density Considerations: Beyond Aqueous Solutions
The conversion of µg/ml to ppm directly as 1:1 (as shown above) is a simplification applicable primarily to dilute aqueous solutions where the density is approximately 1 g/mL. If the solvent has a density significantly different from 1 g/mL, we must account for this density difference. Here’s how:
-
Determine the density (ρ) of the solution: This information is usually provided or can be found in reference tables. The density is expressed in g/ml or kg/L.
-
Convert µg/ml to mg/L: This is a simple conversion; we multiply by 1000 (since 1 mg = 1000 µg and 1 L = 1000 mL):
- 1000 µg/ml * (1 mg/1000 µg) * (1000 ml/1 L) = 1000 mg/L
-
Account for density: In the case of a solution with density 'ρ' g/ml, the mass of 1 liter of solution would be 1000ρ g. Thus, our ppm value would be modified as follows:
ppm = (mass of solute in mg / mass of solution in kg) * 10⁶ = (mass of solute in mg / (volume of solution in L * density in kg/L)) * 10⁶
For our example (1000 mg/L):
ppm = (1000 mg/L) / (ρ kg/L) * 1000
Where ρ is the solution's density in kg/L. If ρ = 1 kg/L (1 g/mL), the formula simplifies to the earlier 1:1 conversion. However, if ρ = 1.2 kg/L, for example:
ppm = (1000 mg/L) / (1.2 kg/L) * 1000 ≈ 833 ppm
In summary: The simple 1:1 conversion (1000 µg/ml = 1000 ppm) is valid for dilute aqueous solutions. For other solutions, the density must be incorporated into the calculation, modifying the final ppm value.
Practical Applications
The conversion of µg/ml to ppm is crucial in many fields:
- Environmental Monitoring: Determining the concentration of pollutants (heavy metals, pesticides) in water or soil samples.
- Food Safety: Measuring the levels of contaminants or additives in food products.
- Pharmaceutical Analysis: Determining the concentration of active ingredients in drug formulations.
- Toxicology: Assessing the toxicity of substances by determining their concentrations in biological samples (blood, urine).
- Industrial Hygiene: Monitoring workplace air for hazardous substances.
Frequently Asked Questions (FAQs)
Q1: What if the density of the solution is unknown?
A1: If the density is unknown, it's impossible to accurately convert µg/ml to ppm. You would need to determine the density using appropriate laboratory methods before proceeding with the conversion.
Q2: Can I always assume a 1:1 conversion between µg/ml and ppm?
A2: No, only for dilute aqueous solutions with a density approximately equal to 1 g/ml. For other solutions, the density must be considered.
Q3: What are the units for ppm in this conversion?
A3: The units for ppm in this context is mass concentration – specifically, milligrams of solute per kilogram of solution (mg/kg). This is because we are often dealing with the mass of the solute relative to the mass of the entire solution. However, as discussed, for dilute aqueous solutions, this is closely approximated by mg/L or µg/mL.
Q4: Are there other units used to express concentration?
A4: Yes, many others exist, including parts per billion (ppb), parts per trillion (ppt), molarity (mol/L), molality (mol/kg), and weight percent (%). Each unit has its own specific application and requires different conversion factors.
Q5: What are some common mistakes to avoid when doing this conversion?
A5: The most common mistake is to assume a 1:1 conversion without considering the density of the solution. Always carefully check the context and ensure the solution density is close to 1 g/ml before applying the simple conversion. Another error is a simple arithmetic mistake during the conversion process. Using a calculator and double-checking the calculations can significantly reduce the risk of errors.
Conclusion
Converting 1000 µg/ml to ppm requires careful consideration of the solution's properties. While a simple 1:1 conversion works for dilute aqueous solutions where the density is approximately 1 g/ml, solutions with different densities require a more involved calculation that includes the density factor. Understanding these nuances is crucial for accurate reporting and analysis in numerous scientific and engineering applications. Always double-check your units and calculations to avoid errors. Accurate concentration determination is foundational to reliable results in a wide array of scientific endeavors. Remember to always consider the context and specifics of your solution when performing this crucial conversion.
Latest Posts
Latest Posts
-
Convert 134 Pounds To Kg
Sep 21, 2025
-
How Big Is 20m Squared
Sep 21, 2025
-
6 Feet By 8 Feet
Sep 21, 2025
-
How Much Is 500 Micrograms
Sep 21, 2025
-
40 Pounds In Aud Dollars
Sep 21, 2025
Related Post
Thank you for visiting our website which covers about 1000 Μg Ml To Ppm . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.